

Kyiv, Symona Petliury St., 28

+38 (096) 515 79 79
+38 (093) 414 79 79
May 13, 2020
All news
According to medical statistics, based on the latest results of epidemiological studies, intact periodontitis was found in only 2-10% of observations, periodontal disease and is observed in 90-95% of the adult population. The increase in the prevalence of generalized periodontal tissue diseases is mainly due to the age of 30 to 50 years, and by 45 years it reaches 96%. Among young people aged 16 to 20, the prevalence of periodontal tissue diseases is increasing sharply and occurs in 50-85% of cases.
The increased prevalence of generalized periodontal tissue diseases, characterized by a long chronic course, with a tendency to periodic exacerbations with further progression of the inflammatory-dystrophic process, which leads to the development of structural and functional disorders of the dentofacial apparatus, is one of the main medical and social problems. In this regard, the development of new approaches to the treatment of generalized periodontitis (hereinafter referred to as “GP”), aimed at achieving long-term stabilization in the course of GP, is one of the priority areas in the development of modern dentistry.
Research in recent years has significantly deepened our understanding of the mechanisms of the emergence and progression of chronic dystrophic-inflammatory periodontal diseases. According to modern ideas, the etiological factors of GP are traditionally divided into local and systemic. Local factors include factors that act directly on periodontal tissues, while systemic factors depend on the general condition of the patient. Among the most significant etiological factors for the development of GP, researchers identify the impact of periodontal pathogenic microorganisms and their waste products on periodontal tissues, on the condition of the oral cavity, which contributes to the formation of dental plaque and affects the periodontal pathogenic potential of microflora, general (systemic) factors that ensure periodontal homeostasis against the background of the existing genetic predisposition, changes in the body’s reactivity. Among the important risk factors for the development of GP are also called disorders of microcirculation and transcapillary metabolism, imbalance of immunocompetent systems, involvement of autoimmunity, and lack of antioxidant protection.
With the “vascular” theory of the pathogenesis of GP – microcirculatory disorders appear already in the early stages of GP and accompany all periods of the disease. They are characterized by inhibition of blood flow velocity, aggregation and stasis of formed blood elements, damage to endothelial cells, which contributes to thrombus formation. The high prevalence of diseases accompanied by endothelial dysfunction (ED), primarily atherosclerosis, contributes to the development of an imbalance between antioxidant defense mechanisms and the rate of CPO. Peroxidation of phospholipids of cell membranes contributes to their destruction, damage to periodontal cells, which in turn reduces the resistance of tissues to damage by etiological factors, including microbial influence.
The accumulation of underoxidized products also contributes to the development of changes in the bone tissue of the alveolar process with the activation of bone resorption processes and the destruction of collagen fibers. Activation of CPO is accompanied by a change in the pH of vascular endothelial cells, periodontal tissues with the development of metabolic acidosis and changes in the properties of cell membranes. Modern ideas about the role of oxidative stress in the pathogenesis of chronic periodontitis allow us to consider the content of lipid peroxidation in saliva and its antioxidant potential as predictors of the escalation of inflammatory periodontal lesions.
Thus, the development of new approaches to the treatment of generalized periodontitis (hereinafter referred to as “GP”), aimed at achieving long-term stabilization in the course of GP, is one of the priority areas in the development of modern dentistry.
Among them is cell engineering, which is based on the use of growth factors produced by many cells, such as platelets, to activate the processes of reparative regeneration of cellular structures.
Already in the 1990s. It has been proven that platelets in our blood contain growth factors – proteins that stimulate the development of stem cells and their transformation into cells of the body tissue that is damaged.
The therapeutic effect of platelet autoplasma (TAP) is explained by the presence of platelets and the content of growth factors (GF) in them, but the effect of blood plasma can also be based on other qualitative components, for exampled micro- and macroelements, vitamins, which are in the most bioavailable state for tissues.
At present, the main goal of research into regeneration processes is the need to identify FRs, knowledge of the mechanism of their action and the possibilities of their application to improve wound surface regeneration.
The use of TAP today represents one of the few opportunities to launch and accelerate natural regeneration mechanisms due to FRs contained in platelets. In addition, it is non-toxic and non-immunoreactive. Obtaining TAP technologically involves the separation of plasma and platelets from erythrocytes both along a density gradient and using specialized laboratory filters.
TAP modulates and regulates the function of primary, secondary and tertiary FRs, affecting all stages of regeneration simultaneously. The mentioned property distinguishes platelet autoplasma FR from recombinant FR, which are responsible for a separate regeneration mechanism.
Phromocytes contain various FRs and cytokines that contribute to the restoration of damaged tissues. In the α-granules of platelets there are more than 30 FRs that affect the processes of periodontal tissue regeneration simultaneously. The most important are: IGF (insulin-like FR) – which promotes stem cell differentiation, increases bone tissue metabolism and collagen production. PDGF (platelet FR) – stimulates the proliferation and migration of mesenchymal (osteogenic) cells, stimulates angiogenesis. PDEGF (platelet FR of endothelial cells) – causes a stimulating effect on endothelial cells and has an angiogenic effect. VEGF or PDAF (vascular endothelial growth factor) – there are 4 types of factor: VEGF-A, -B, -C and -D. They participate in angiogenesis, induce proliferation of vascular endothelial cells. EGF (epidermal growth factor) – promotes proliferation of fibro- and osteoblasts, increases fibronectin synthesis. TGF-ß (“Family” of transforming growth factors) – multifunctional factors, since they not only induce differentiation of mesenchymal cells, but also cause a large number of cellular and intercellular responses, including the production of other growth factors. Transforming growth factors include bone morphogenetic proteins, some of which (BMP-2, osteogenic or BMP-3, BMP-4,-5,-7,-8 and-9) are pronounced osteoinducers, modulate cell proliferation and differentiation of poorly differentiated cells into osteoblasts. PLGF-1 / -2 (placental FR) – potentiate the action of VEGF, increase the permeability of the vascular wall. FGF (fibroblast FR) – causes expression in bone tissue, angiogenesis, osteogenesis, induces the production of TGF in osteoblast cells. Osteonectin – “cultural shock protein”: makes up 15% of the organic component of the bone matrix, regulates proliferation and interaction of cells with the matrix. Thrombospondin – mediates the adhesion of bone cells. FR are delivered to the tissues in the form of autoplasma injection and are concentrated by introducing a larger amount of autoplasma – this increases the activity of fibroblasts and stimulates their formation. Fibroblasts produce collagen fibers, hyaluronic acid and elastin. All this leads to the formation of new connective tissue, the growth of capillaries. FR also block osteoclasts and stimulate osteoblast proliferation, which further inhibits this decrease in bone tissue and promotes its regeneration. As a result, metabolic processes are restored, microcirculation and metabolism in tissue cells are improved, tissue respiration is normalized, and local immunity is activated.
Contraindications to the use of the method are: malignant neoplasms; systemic blood diseases, mental illnesses, allergic reaction to anticoagulant (sodium heparin) in history.
For the treatment of patients, autologous plasma obtained by the following methods was used:
1)Obtaining platelet-rich plasma PRP (Platelets Reach Plasma, Lifting-Plasma), which will NOT thicken in the future. An anticoagulant is used to use PRP plasma. It is used for PRP therapy. The method of obtaining PRP assumes that the plasma will NOT clot either before or after its introduction into the patient’s body. The mechanism of action is the same growth factors.
When obtaining platelet-rich plasma, it is appropriate to use tubes containinganticoagulant ACD – A. The choice of anticoagulant is not accidental. It is this anticoagulant that allows to ensure maximum survival and activity of platelets. In blood banks, this anticoagulant is used to store platelet mass. During a single centrifugation in a test tube, we see a separation into an erythrocyte-leukocyte layer (dark) and plasma. Sometimes there is a pronounced whitish layer between them, it is called Buffy Coat – this is a fraction of an anticoagulated blood sample, which contains most of the leukocytes and platelets after centrifugation in a blood density gradient.
2) PRF (Platelets Reach Fibrin) – platelet-rich fibrin, no anticoagulant is used in the test tube:
-a. A-PRF (advance, i.e. “improved”, PRF-clots) – obtaining platelet-rich plasma, which thickens before use (anticoagulant is not used). This is a thickened part of blood plasma, which contains many growth factors for osteo and angiogenesis. From them, membranes and plugs can be prepared using a special PRF-BOX box. The essence of PRF-BOX is to squeeze fibrin in order to free it from excess plasma. Membranes are used to cover bone materials, palate defects after taking gum grafts, Schneider’s membrane when it is perforated and simply sinus lifts, used for periodontal surgery. Plugs – to fill post-extraction tooth holes and periodontal defects. Similarly, clots can be cut into small pieces with scissors and mixed with bone substitutes.
-b. i-PRF (injectable – injectable) – obtaining platelet-rich plasma, which thickens after application. Such plasma is used for mixing with bone substitute, in order to stabilize it and give it a certain shape. For injections into periodontal tissues to improve their blood supply, change the biotypes of the gums. Sometimes for gluing the edges of the mucous membrane, for example, Schneider’s membrane.
Actation of platelets in response to tissue damage occurs during the PRF release process of several biologically active proteins, including; platelet alpha granules, platelet-derived growth factor (PGDF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF) and epidermal growth factor. In fact, platelet and leukocyte cytokines play an important role in the role-playing of this biomaterial, but their support fibrin matrix is very useful for forming the defining elements responsible for the real therapeutic potential of PRF. Cytokines are immediately used and destroyed in the healing wound. The harmony between cytokines and their support by the fibrin matrix is much more unique than any other platelet derivative.
3) PPP – Platelet Poor Plasma – plasma with a “normal” platelet concentration. In the tubes with a green sticker there is initially a separating gel. This is convenient because the gel after centrifugation rises and tightly fixes the erythrocyte-leukocyte clot, preventing mixing with plasma, BUT the centrifugation parameters for tubes containing the gel do not allow the use of low centrifugation speeds and in this regard the final product – plasma, cannot contain high concentrations of platelets, however the content of proteins, amino acids, minerals and everything else remains unchanged, which gives us the opportunity to use this form almost everywhere, as it restores all impaired tissue functions.
Today, there is a wide arsenal of methods and tools developed for the complex treatment of generalized periodontitis and chronic catarrhal gingivitis, while achieving stable stabilization of the pathological process in periodontal tissues is difficult. The issue of determining the optimal approach to the treatment of this category of patients is quite relevant and requires further research. Treatment of periodontal diseases is important not only for maintaining oral health, but, as the analysis of literature shows, and for reducing pathological changes in periodontal tissues, which will make it possible to develop a new comprehensive pathogenetically based approach to the treatment of generalized periodontitis and chronic catarrhal gingivitis, aimed at stable stabilization of the dystrophic-inflammatory process in periodontal tissues, which will simultaneously contribute to strengthening the health of patients as a whole.
The study involved 27 people, including 13 men and 14 women aged 25 to 65 years, with diagnoses: chronic catarrhal gingivitis of moderate severity, chronic generalized periodontitis of initial-I and II degrees of severity. All patients were divided into 2 groups: control – 12 people and main – 15 patients. Treatment of gingivitis and periodontitis included treatment of caries and its complications.nen; teaching patients oral hygiene and its control; conducting professional hygiene and closed curettage. General anti-inflammatory therapy was prescribed to patients diagnosed with generalized periodontitis and was carried out using antibacterial drugs. Traditional therapy was supplemented with the appointment of Biogen Prodentis, resorbable 3-4 times a day for 10 days. In the main group: for gingivitis and initial-I degree of periodontitis after professional hygiene, for periodontitis – after closed curettage, – iPRF was introduced into the transitional fold area. A total of 3 procedures were performed with an interval of 10 days, the next 2 procedures were administered – PPP. Evaluation of results: after each procedure – a control examination; the next visit – after 6 months. If necessary, after 6 months, maintenance therapy was performed – a course of professional hygiene with 1 PPP procedure (if necessary).
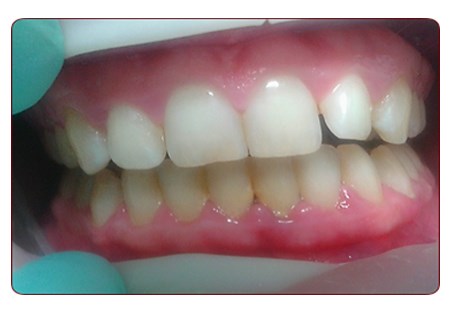
Photo 1. Patient M., 28 years old. Diagnosis: chronic catarrhal gingivitis of moderate severity. Before treatment.

Photo 2. Patient M., 28 years old. Diagnosis: chronic catarrhal gingivitis of moderate severity. Immediately after treatment: removal of dental plaque and the first plasma therapy procedure – iPRF.

Photo 3. Patient M., 28 years old. Diagnosis: chronic catarrhal gingivitis of moderate severity. 3 years after treatment.

Photo 4. Patient 32 years old. Generalized periodontitis of mild severity. Dental deposits. before treatment

Photo 5. Patient 32 years old. Generalized periodontitis of mild severity. Dental deposits. Immediately after scaling and I-PRF injections.

Photo 6. 32-year-old patient. Mild generalized periodontitis. Dental deposits. 5 days after treatment.
A week after the start of treatment, patients in the main group who were injected with platelet autoplasma showed positive clinical dynamics, which was expressed in an improvement in hygienic and periodontal indices by 40%, a decrease in gum bleeding by 60%, and a decrease in gum hyperemia. In the control group of patients, complaints of gum bleeding and limited papillary gum hyperemia persisted in 70% of cases during this period. The index values did not differ from the baseline level.
After 6 months after treatment, patients in the II (main) group completely recovered from signs of periodontal inflammation: there was no bleeding, swelling, or gum hyperemia, and the probing depth of periodontal pockets decreased. In the control group of patients, gingival bleeding decreased by 50% compared to baseline values. Swelling of the papillary gingival margin and the depth of periodontal pockets remained at baseline.
After 12 months, the clinical condition of the periodontium in the patients of the main group was stable, which was confirmed by the absence of an increase in periodontal index indicators and restoration of tooth function. In the control group, the index values characterizing the condition of the periodontium remained 2.5 times higher than in the comparison group.
The results of the study showed the effectiveness and, accordingly, the validity of the use of platelet autoplasma in the complex treatment of periodontal diseases. A decrease in the index values characterizing the condition of the periodontium was noted in the II group of patients already up to 7 days after the start of treatment. Similar indicators in the control group during this period practically did not differ from the baseline. 12 months after treatment, the decrease (normalization) of periodontal indices in group II occurred by 82%, and in group I (control) – only by 65%.
Conclusion: The inclusion of platelet autoplasma in the complex treatment of periodontitis allows treating inflammation and stabilizing the process in a shorter time compared to conventional treatment methods.